electron configuration of hydrogen|electron configuration for every element : Baguio How to Write the Electron Configuration for Hydrogen. Since Hydrogen only has one electron it is the simplest electron configuration to write. Essentially there is just one electron around the Hydrogen nucleus. Video: Hydrogen Electron Configuration Notation. No deposit casino bonus codes for existing players in 2023 offer a unique, risk-free opportunity to enhance gaming and win potential rewards. The key lies in a strategic approach, from choosing games with higher odds to .
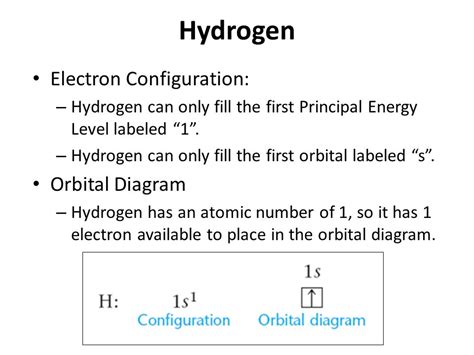
electron configuration of hydrogen,How to Write the Electron Configuration for Hydrogen. Since Hydrogen only has one electron it is the simplest electron configuration to write. Essentially there is just one electron around the Hydrogen nucleus. Video: Hydrogen Electron Configuration Notation.electron configuration of hydrogen electron configuration for every elementHelium only has 2 electrons and therefore it has a configuration of 1s 2. Because the . Mar 23, 2023 For hydrogen, therefore, the single electron is placed in the 1s orbital, which is the orbital lowest in energy (Figure \(\PageIndex{1}\)), and the electron configuration is written as .

Thus, because a hydrogen atom has its single electron in the s subshell of the first shell, we use 1s 1 (spoken as “one-ess-one”) to describe the electron .By “building up” from hydrogen, this table can be used to determine the electron configuration for any atom on the periodic table. We will now construct the ground-state electron configuration and orbital diagram . An atom of hydrogen (atomic number 1) has one proton and one electron. The single electron is assigned to the 1s sublevel, the lowest-energy sublevel in the lowest .Electron Configuration of Hydrogen The atomic number of hydrogen is 1. Therefore, a hydrogen atom contains 1 electron, which will be placed in the s subshell of the first shell/orbit.Learn about the electron configurations of atoms, including hydrogen, and how to use the Aufbau principle and the Pauli exclusion principle. This web page has a glitch and .
This web page is part of a free textbook on organic chemistry, but it has a glitch and cannot be accessed. It does not contain any information about the electron configuration . Electron configurations describe where electrons are located around the nucleus of an atom. For example, the electron configuration of lithium, 1s²2s¹, tells us .The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first .Electron atomic and molecular orbitals A Bohr diagram of lithium. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s 2 2s 2 2p 6, meaning that the 1s, 2s, and .
This structure is called an electron configuration and is unique to hydrogen. Helium atoms have 2 electrons. Both electrons fit into the 1s subshell because s subshells contain one s orbital which can hold up to 2 electrons; therefore, the electron configuration for helium atoms is 1s 2 (spoken as “one-ess-two”). Figure 8.7.1 8.7. 1: Electron configuration of a ground-state hydrogen atom depicted on an energy-level diagram. The electron is represented by an arrow in the 1s orbital. On the energy level diagram in Figure 8.7.1 8.7. 1, the horizontal lines labeled 1s, 2s, 2p, etc. denote the spatial parts of the orbitals, and an arrow pointing up for spin . Electron configurations describe where electrons are located around the nucleus of an atom. For example, the electron configuration of lithium, . So elements in period 1 .

Introduction. The electron configuration is the standard notation used to describe the electronic structure of an atom. Under the orbital approximation, we let each electron occupy an orbital, which can be solved by a single wavefunction. In doing so, we obtain three quantum numbers (n,l,m l), which are the same as the ones obtained from . Helium atoms have 2 electrons. Both electrons fit into the 1s subshell because s subshells can hold up to 2 electrons; therefore, the electron configuration for helium atoms is 1s 2 (spoken as “one-ess-two”). Different subshells hold a different maximum number of electrons. Any s subshell can hold up to 2 electrons; p, 6; d, 10; .electron configuration of hydrogenHydrogen, for instance, has only one electron, which must occupy the lowest-energy orbital. Thus, hydrogen has a 1s ground-state configuration. Carbon has six electrons and the ground-state configuration 1s 2 2s 2 2p x 1 2p y 1, and so forth. Note that a superscript is used to represent the number of electrons in a particular orbital.
electron configuration for every elementHydrogen, for instance, has only one electron, which must occupy the lowest-energy orbital. Thus, hydrogen has a 1s ground-state configuration. Carbon has six electrons and the ground-state configuration 1s 2 2s 2 2p x 1 2p y 1, and so forth. Note that a superscript is used to represent the number of electrons in a particular orbital.
electron configuration of hydrogen|electron configuration for every element
PH0 · the correct electron configuration for te is
PH1 · hydrogen complete electron configuration
PH2 · how many electrons does hydrogen have
PH3 · electron configuration periodic table
PH4 · electron configuration for every element
PH5 · electron configuration chart
PH6 · electron configuration calculator
PH7 · complete the electron configuration for f
PH8 · Iba pa